ascl3 electron geometry|8.6: Molecular Geometries : iloilo After calculating the electronic geometry from VESPR we can determine the molecular geometry based on the bonding orbitals. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the . ww1.rapbhe.net ist eine alternative Domain für rapbhe.net, eine Plattform für Rap-Musik und Hip-Hop-Kultur.
ascl3 electron geometry,Abr 25, 2015 — Learn how to determine the molecular geometry of AsCl3 using VSEPR theory and Lewis structure. See the answer, explanation and video from Socratic, a chemistry Q&A platform.AsCl3 is a pyramidal molecule with C3v symmetry. The As-Cl bond is 2.161 Å and the angle Cl-As-Cl is 98° 25'±30. AsCl3 has four normal modes of vibration: ν1(A1) 416, ν2(A1) 192, ν3 393, and ν4(E) 152 cm .ascl3 electron geometryAgo 10, 2013 — A step-by-step explanation of how to draw the AsCl3 Lewis Dot Structure ( Arsenic trichloride). For the AsCl3 structure use the periodic table to find the total number of valence electrons f.
8.6: Molecular Geometries After calculating the electronic geometry from VESPR we can determine the molecular geometry based on the bonding orbitals. If there are no lone pairs and all orbitals are bonding, then the molecular geometry is the .Ago 4, 2022 — To determine the electron geometry, we use the VSEPR (Valence Shell Electron Pair Repulsion) theory. According to VSEPR, the electron groups around the .The Lewis structure for AsCl 3 is similar to AsF 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their .
IUPAC Standard InChI:InChI=1S/AsCl3/c2-1 (3)4 Copy. IUPAC Standard InChIKey:OEYOHULQRFXULB-UHFFFAOYSA-N Copy. CAS Registry Number: 7784 .
ChemSpider record containing structure, synonyms, properties, vendors and database links for Arsenic trichloride, 7784-34-1, 232-059-5.Hun 23, 2023 — In order to find the total valence electrons in AsCl3 molecule, first of all you should know the valence electrons present in arsenic atom as well as chlorine atom. (Valence electrons are the .Lewis electron structures give no information about molecular geometry, the arrangement of bonded atoms in a molecule or polyatomic ion, which is crucial to understanding the chemistry of a molecule. The valence-shell electron-pair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases .Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well.Dis 16, 2021 — Table 1.1 Basic VSEPR Shapes. Notes: . For VSEPR purpose, the terms “shape” and “geometry” are interchangeable; “electron pair” and “electron group” are also interchangeable. Multiple bonds .Question: What is the molecular geometry of AsCl3? T-shaped tetrahedral trigonal planar trigonal pyramidal. What is the molecular geometry of AsCl 3? T-shaped. tetrahedral. trigonal planar. trigonal pyramidal. There’s just one step to solve this. 100 % (5 ratings) Step 1. Given:- AsCl3 . As -electronic configuration=[Ar] 3d¹⁰ 4s² 4p³ .The Electron Pair Geometry of a molecule is determined by the total number of electron pairs around a central atom. Electron pairs are the bonded electrons, lone pairs and single unpaired electrons. Total number of electron pairs = ½ X [(number of electron pairs on central atom) + (number of monovalent atoms on the central atom) + (anionic .ascl3 electron geometry 8.6: Molecular Geometries IUPAC Standard InChIKey: OEYOHULQRFXULB-UHFFFAOYSA-N Copy CAS Registry Number: 7784-34-1 Chemical structure: This structure is also available as a 2d Mol file; Other names: Arsenic(III) chloride; AsCl3; Arsenous chloride; Arsenous trichloride; Arsenic chloride; Arsenic butter; Arsenious chloride; Butter of arsenic; Chlorure arsenieux; .Molecular Geometry; Hybridization; Polarity; Resonance Structures; Ionic and Covalent Bonds; Practice! Drawing the Lewis Structure for AsCl 3 (Arsenic Trichloride) Viewing Notes: The Lewis structure for AsCl 3 is similar to AsF 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their .Hul 20, 2019 — Electron domain is used in VSEPR theory to determine the molecular geometry of a molecule. The convention is to indicate the number of bonding electron pairs by the capital letter X, the number of lone electron pairs by the capital letter E, and the capital letter A for the central atom of the molecule (AX n E m).When predicting .Ago 14, 2020 — As such, this model of molecular geometry is often referred to as the valence shell electron pair repulsion (VSEPR) theory. For reasons that will become clear, extension of this model implies that a better name is the Electron Domain (ED) Theory. This model also accounts, at least approximately, for the bond angles of \(\ce{H_2O}\) .

Predicting Electron Pair Geometry and Molecular Structure. The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures: Draw the Lewis structure of the molecule or polyatomic ion. Count the number of regions of electron density (lone pairs and bonds) around the central atom.Set 10, 2015 — The idea here is that you have two kinds of geometries, molecular geometry and electron geometry. Molecular geometry does not take into account any lone pairs that are present on the central atom. In the case of arsenic trichloride, the central arsenic atom has one lone pair of electrons presentThe valence shell electron pair repulsion theory is a bonding theory that can be used to predict the shape or geometry of a molecule. The geometry of a molecule is determined by looking at the number of bonding and nonbonding groups of electrons around the central atom. Answer and Explanation: 1May 26, 2021 — An explanation of the molecular geometry for the AsF3 (Arsenic trifluoride) including a description of the AsF3 bond angles. The electron geometry for the Ar.

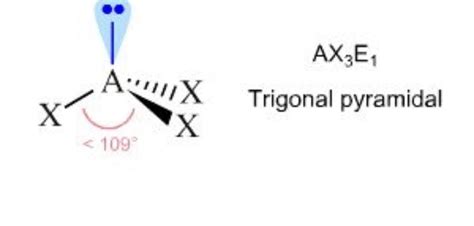
The VSEPR theory (Valence Shell Electron Pair Repulsion theory) can help us predict the molecular geometry based on the number of bonding and lone pairs around the central atom. According to VSEPR theory, when a central atom has three bonding pairs and one lone pair, the molecular geometry is trigonal pyramidal.
Nob 25, 2020 — An explanation of the molecular geometry for the SCl2 (Sulfur dichloride) including a description of the SCl2 bond angles. The electron geometry for the Sulf.Ago 4, 2022 — To determine the electron geometry, we use the VSEPR (Valence Shell Electron Pair Repulsion) theory. According to VSEPR, the electron groups around the central atom will arrange themselves in a way that minimizes repulsion. In AsCl3, there are three chlorine atoms bonded to the central arsenic atom.
Ago 10, 2013 — A step-by-step explanation of how to draw the AsCl3 Lewis Dot Structure ( Arsenic trichloride).For the AsCl3 structure use the periodic table to find the tot.1 day ago — Electron geometry is tetrahedral, and shape is trigonal pyramidal. AsH3 Hybridization. The geometry and bonding of some polyatomic covalent compounds are explained using a unique concept called hybridization. Hybridization is the mixing of atomic orbitals to form equivalent hybrid orbitals. It involves the redistribution of energy.
ascl3 electron geometry|8.6: Molecular Geometries
PH0 · What is the molecular geometry of AsCl3? tetrahedral trigonal
PH1 · What is the molecular geometry of AsCl3 ? A) tetrahedral B
PH2 · What is the molecular geometry of AsCl3 ? A)
PH3 · Lewis Structure of AsCl3 (With 6 Simple Steps to Draw!)
PH4 · AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure for
PH5 · AsCl3 Lewis Structure: How to Draw the Lewis Dot Structure
PH6 · AsCl3 Lewis Structure in 6 Steps (With Images)
PH7 · AsCl3 Lewis Structure in 6 Steps (With Images)
PH8 · AsCl3 Lewis Structure
PH9 · Arsenic trichloride
PH10 · 8.6: Molecular Geometries